Many things in the renewable energy space have been alive solely because of the government policies and subsidies. Some, like solar power for example, have fully fledged over time, crossed an important “grid parity” threshold on the cost side, and now compete with the traditional energy producers. Others, like traditional biofuels, have stayed uncompetitive on the cost side, and will likely stay there forever.
Land-grown biofuels as fuels
The reason why biofuels produced from land-grown biomass are uncompetitive as fuels (that is as a source of energy), is because it takes a lot of land to produce a unit of energy. Traditional land-grown biofuels rely on conversion of sunlight energy into chemical energy – natural photosynthesis – the process of structurally low efficiency.
Here is some arithmetics:
- Assume average insolation of about 225 W/m2
- Sugarcane, one of the most photosynthesis-efficient crops, produces biomass energy-worth of about 0.86 W/m2 (thus solar-to-biomass efficiency is only 0.86/225=0.38%)
- Not all of this biomass can be converted into fuels. Further processing into ethanol, for example, results in solar-to-fuel efficiency of 0.13%
- Note that traditional internal combustion engines (that burn traditional fuels, as well as biofuels) have efficiency of about 25%, thus solar-to-motion conversion rate is about 0.03%
- For comparison, average efficiency of a standard single layer solar PV panel (solar-to-electricity) is about 20%, which, taking into account that electric engines are 90%+ efficient, is more than 500x higher than solar-to-motion conversion via biofuels
If you have a piece of land and consider the following two options – grow plants and convert it into biofuels, or install solar panels to generate electricity – the latter will be by far much more economic: the two activities have different CapEx and OpEx, furthermore the price of liquid fuels is different than the price of electricity, but this huge several orders of magnitude difference in the yield makes the conclusion straightforward – harvesting electricity via solar panels is much more profitable than harvesting plants for fuels.
Land-grown biofuels are non-competitive, and will never be.
Non land-grown biofuels as fuels
Second generation biofuels are produced from agricultural residues, municipal waste, etc. They don’t compete for land space, and as such the unfavorable comparison against the solar panels (described above) is not applicable.
The economics of some of these technologies may be reasonable:
- you are paid(!) a fee to take unwanted feedstock (e.g. tipping fee for municipal waste disposal)
- apply proper chemical conversion technology
- obtain a marketable product (synthetic crude oil, diesel, etc)
What most of such technologies have in common is that they decompose biomass into simple constituent parts (like syngas, a mixture of CO and H2) and build more complex organic molecules in a bottom-up fashion, unlike traditional oil-based chemistry that refines oil top-down.
BioFuels => BioChemistry
Second generation biofuels may well be competitive in a wide range of economic environments, but the true potential of the technology is not to produce fuels (complex organic molecules burnt only to extract energy contained in their chemical bonds), but rather to be the foundation of renewable chemistry that will eventually replace traditional oil-based chemistry.
Organic chemistry is based on C (carbon) and H (hydrogen). Thus, renewable organic chemistry would require a sustainable source of both.
Several approaches are possible.
1) Traditional, top down, petrochemistry is based on crude oil feedstock. Crude oil, a rich mixture of various types of hydrocarbons, is refined into different hydrocarbon fractions that are further converted into various chemical materials.
2) Emerging, bottom up, approach is based on biomass feedstock. It goes through the following stages:
- biomass is converted into syngas ( CO + H2 )
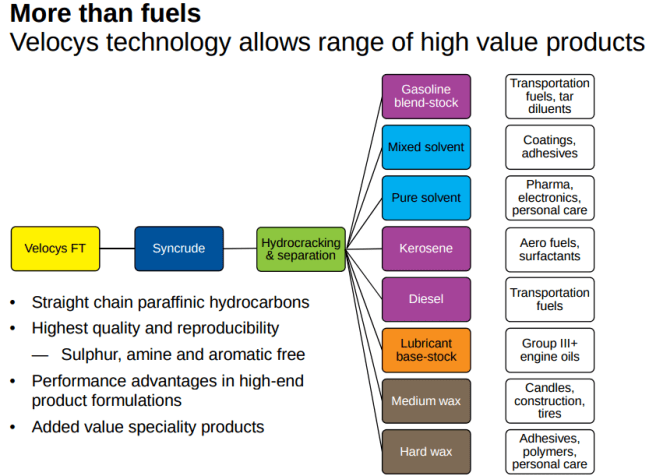
- syngas, through Fischer-Tropsch process, yields a wide range of chemical products (see picture below)
Source: Velocys
3) Another emerging, bottom up, approach – artificial photosynthesis (replication of the nature’s conversion of water and carbon dioxide into something more useful).
CO2 + H2O + energy (sunlight) + catalysts (genetically engineered bacteria) => various chemical products
Source: Joule Unlimited
Traditional oil-based chemistry takes a ready mix of hydrocarbons and refines it into useful products. The new approaches (2) and 3) above) take C and H in very simple forms, and build the desired products bottom up.
BioMass as a source of carbon
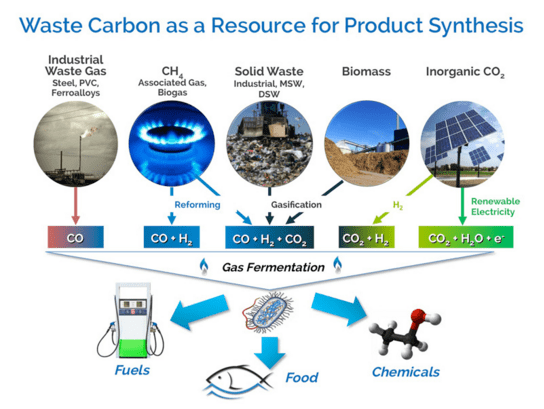
One problem with the new approaches is that they require a concentrated source of C (and H, but H is abundant in the form of water, and hence is not a problem). Concentration of CO2 in the air, for example, is mere 0.04%.
One way to tackle this problem is to locate new chemistry production at the existing industrial facilities where concentrated CO2 is emitted in the atmosphere (steel mills, etc).
Another way is to (yet again) take inspiration from nature, that takes low concentration C from the air and builds it up in biomass through natural photosynthesis. Land-grown biofuels, as sources of energy, were stillborn, but growing concentrated C as feedstock for renewable chemistry is a completely different story. What a turn.
Source: LanzaTech
With the recent technological advancements, electricity is likely to become the dominant energy form in the transportation sector within a few decades. That would leave oil to compete with biomass in the chemistry industry.